
- #REACTIVITY TABLE OF ELEMENTS FULL#
- #REACTIVITY TABLE OF ELEMENTS ANDROID#
- #REACTIVITY TABLE OF ELEMENTS SERIES#
#REACTIVITY TABLE OF ELEMENTS SERIES#
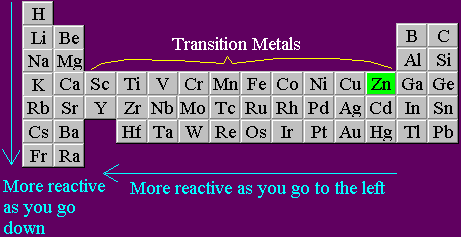
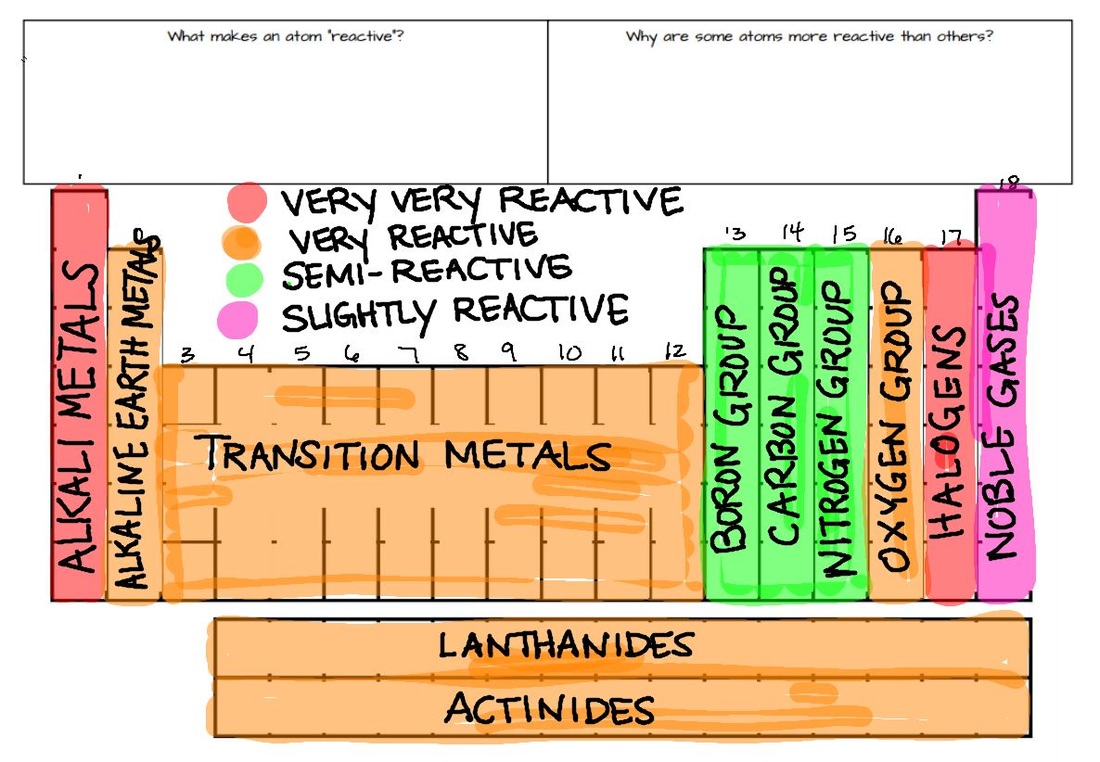
The electro-positivity (tendency to lose electrons) of the elements gets reduced while moving down the reactivity series of metals, as we go down in the series it’s hard for elements to lose electrons, hence the less reactivity.For example, sodium due to its high reactivity explodes as came in contact with air or water, thus needs to be kept away from moisture and air, and dipped in kerosene. These metals can get tarnished or corrode very easily. The reducing tendency of metals at the top of the table has high, which is why they are easily oxidized.

ISRO CS Original Papers and Official Keys.Interactive Periodic Table Periodic Trends Made Easy. GATE CS Original Papers and Official Keys The reactivity series is a hierarchical arrangement of elements based on their relative tendency.(the alkali metals) in the periodic table, the elements get more reactive. DevOps Engineering - Planning to Production With in the halogen the more reactive elements are displayed at the top where.Python Backend Development with Django(Live).
#REACTIVITY TABLE OF ELEMENTS ANDROID#
#REACTIVITY TABLE OF ELEMENTS FULL#
Full Stack Development with React & Node JS(Live).Java Programming - Beginner to Advanced.Data Structure & Algorithm-Self Paced(C++/JAVA).Data Structures & Algorithms in JavaScript.Data Structure & Algorithm Classes (Live).Fluorine’s unique feature has not only made it the anchor point of the well-known Pauling electronegativity scale, but it has also changed the reactivity and properties of fluorine-containing compounds and materials as compared to non-fluorinated ones. It has a distinct odor that is distinct from the other halogens, chlorine (Cl), bromine (Br), and iodine (I).įluorine is the most electronegative element, which means that when it is connected to another element, it draws electron density towards its own core. In fact, it’s an element of extremes with a wide range of uses, and it could surprise us in the future.įluorine (atomic symbol: F) is the lightest member of the halogen group, commonly known as group 17 in the Periodic System of Elements, and the 13th most prevalent element in the earth’s crust.įluorine is a diatomic gas with a yellowish hue under normal conditions. Cesium is the most reactive element since it is the second from the bottom of this group, has six electron shells, and exhibits the characteristics of a reactive atom.įluorine is a noteworthy chemical element for many reasons, not the least of which is its unusual reactivity. The most reactive substance – Alkali metals are the most reactive element group (situated far apart from intermediate metals and noble gasses). The ability of metals to donate electrons decreases as they progress through the activity series, which is an essential property. It can also be used to determine metal reactivity in the presence of water and acids. The reactivity series data can be used to determine if a metal can displace another in a single displacement reaction. These metals discolor and corrode quickly. Because they are easily oxidized, the metals near the top of the reactivity range are powerful reducing agents. The activity series, sometimes known as the reactivity series, is a list of metals arranged in descending order of their activities. Let’s start with the most reactive element and the reactivity series. The reactivity series is used to determine an element’s reactivity. Answer: Cesium is the most reactive element since it is the second from the bottom of this group, has six electron shells, and exhibits the characteristics of a reactive atom.


 0 kommentar(er)
0 kommentar(er)
